By Michael Teodorov and Sandra Robinson
Find the Q-Tip below! There is a Q-Tip hidden in this article. Find it by reading the article and clicking on the link. Once you find it, the Q-Tip will automatically download so you can save it to your collection. .
Click here to test your knowledge and complete a survey for a chance to win a gift card.
Women’s College Hospital (WCH) has numerous processes for ensuring that safe and effective care is provided as well as the provision of a safe environment for its staff, physicians, learners, volunteers, and visitors. A robust Preventative Maintenance (PM) program for medical devices and medical equipment is an important part of the hospital’s safety program.
Biomedical Engineering is responsible for managing all biomedical equipment at WCH, excluding Diagnostic Imaging and Core Lab. They focus on patient and staff safety, effective use of technology, cost reduction, medical technology support, and compliance with all applicable standards and regulations including Accreditation Canada’s Required Organizational Practice (ROP) related to a Preventative Maintenance Program. Biomedical Services at WCH are provided by Christie Innomed who are available 24 hours/day to respond to maintenance requests. Click here for the QTip.
Preventative maintenance is the process of performing regularly scheduled maintenance activities to help prevent unexpected failures. All medical equipment and devices at WCH are assigned to a preventive maintenance inspection strategy and schedule. PM inspections are interval-based, and their frequency is determined based on manufacturer recommendations and risk levels of the activity and equipment involved.
But that’s not all that Biomedical Engineering does – they are also involved in the following:
- Selection and acquisition of medical equipment: Biomedical Engineering actively participates in the selection and acquisition of equipment, including but not limited to, assistance with coordinating construction activities to prepare the site for the equipment, coordinating installation efforts, performing acceptance testing, and coordinating training of clinical staff.
- Evaluation of equipment for inclusion into the Equipment Management Program: All equipment that directly supports patient care, or that is located within the patient care setting, regardless of ownership, is assessed for inclusion into the Equipment Management Program. Medical equipment receives appropriate maintenance, with the ultimate goal of keeping equipment safe, reliable, and performing according to specifications while using resources efficiently.
- Incoming inspections of medical equipment: All hospital owned equipment that directly supports patient care at WCH, or that is located within the patient care setting, regardless of acquisition method, is inspected prior to initial use. Equipment is inspected to assure physical condition, functionality, accuracy, patient/operator safety, electrical safety, and proper documentation.
- Repairs: All repairs are prioritized and performed in a timely manner.
- Review and follow-up medical devices hazard alerts and product recalls: Biomedical Engineering also monitors hazard alerts and product recalls from manufacturers, vendors, ECRI, Health Canada, US Food and Drug Administration, etc.
What should staff do if they discover a medical device or medical equipment that is broken or not functioning properly?
- Remove the device or equipment from circulation.
- Contact Biomedical Engineering at 416-323-6029 (after hours call 1-866-888-0885) or Bio.Med@wchospital.ca.
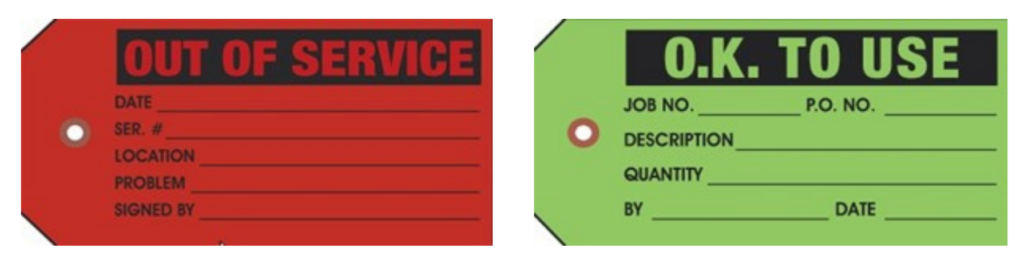
- Label the device or equipment with a red “Out of Service” repair tag to communicate it is out of service.
When the medical device or medical equipment is repaired and back in service, it is labelled with a green “O.K. To Use” tag.
To learn more about Accreditation, visit the Accreditation Intranet Hub.